TO OUR EU CLIENTS:
Dear Clients, if you are ordering from inside the EU, please order through our Irish site https://www.franklpharma.ie/ to avoid post-Brexit VAT charges. If you are based in the UK, please order as usual through this site. And please accept our apologies for any delays.An analysis of eight European studies into the efficacy of Soratinex (2001 to 2016)
TOTAL PATIENTS: 2050
- 46 non-compliant and discontinued treatment (2%)
Of those who continued treatment
- Outstanding improvement (76-100%) in 881 patients (43%)
- Good improvement (51-75%) in 852 patients (42%)
- Moderate improvement (26-50%) in 184 patients (9%)
- Ineffective (0-25%) in 59 patients (3%)
- 28 patients worsened and discontinued (1%)
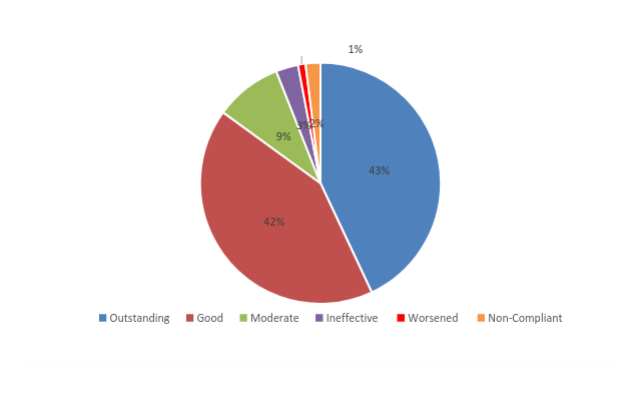
(Source: Journal of Biological Regulators & Homeostatic Agents, April-June 2016. Analysis: FRANKL Pharma. All studies took place between 2001 and 2016).
https://www.ncbi.nlm.nih.gov/pubmed/27498651 - A multi-centred open trial of “Dr Michaels®” (also branded as Soratinex®) topical product family in psoriasis.
https://www.ncbi.nlm.nih.gov/pubmed/27498668 - Efficacy and safety of Dr Michaels® (Soratinex®) product family for the topical treatment of psoriasis: a monitored status study.
https://www.ncbi.nlm.nih.gov/pubmed/27498655 - Clinical evaluation of the effectiveness of “Dr Michaels®” (also branded as Soratinex®) products in the topical treatment of patients with plaque psoriasis.
https://www.ncbi.nlm.nih.gov/pubmed/27498669 - Quality of life aspects of patients with psoriasis using a series of herbal products.
https://www.ncbi.nlm.nih.gov/pubmed/27498667 - An innovative, promising topical treatment for psoriasis: a Romanian clinical study.
https://www.ncbi.nlm.nih.gov/pubmed/27498666 - Scalp psoriasis: a promising natural treatment.
https://www.ncbi.nlm.nih.gov/pubmed/27498657 - Dr Michaels® (Soratinex®) product for the topical treatment of psoriasis: a Hungarian/Czech and Slovak study.
https://www.ncbi.nlm.nih.gov/pubmed/27498653 - A clinical examination of the efficacy of preparation of Dr Michaels® (also branded as Soratinex®) products in the treatment of psoriasis.
